THE COMMITMENT TO RESEARCH
Since 1996, Erchonia, the manufacturer of Lunula, has been committed to fully elucidating the medical utility of low level laser therapy through rigorous clinical studies. For almost 2 decades, Erchonia has studied the clinical utility of low level laser devices for the treatment of numerous medical ailments. Their recent device, Lunula, looks to revolutionize the standard of care for onychomycosis.
Lunula has been markedly studied – from the early in-vitro analysis to the extensive in-vivo studies – and its clinical utility to target painful and unsightly toenail infections has been substantiated. The unique dual-diode approach of Lunula effectively targets the causative infectious agent while fortifying the body’s natural defense mechanisms. This multifaceted approach is the first of its kind, providing patients with a truly effective, yet safe, option for onychomycosis.
As you will quickly learn, Lunula is supported by an unwavering clinical foundation of both histological and clinical evidence that upholds the viability of this approach and ensures an effective option for your patients suffering with onychomycosis. In fact, Lunula is so unique, Erchonia has filed multiple method and device patents specific to Lunula.
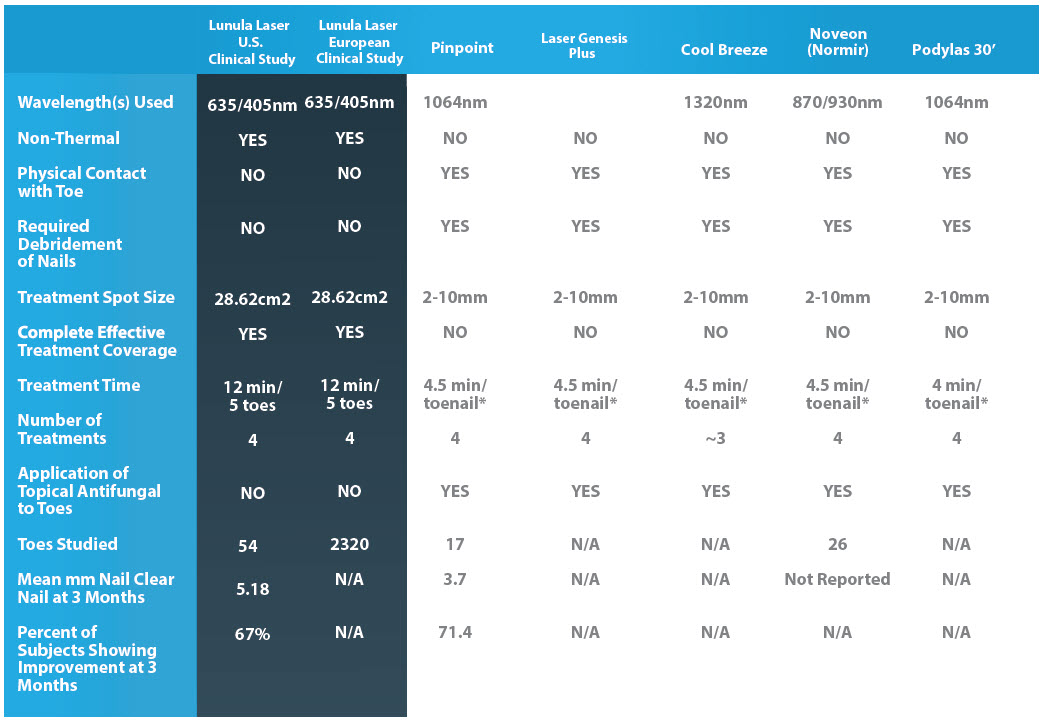
The United States study consisted of 54 great toenails subject to laser irradiation at 405nm and 635nm for twelve minutes at weekly intervals for four weeks. This study was used to obtain the Lunula Laser 510(k) FDA market clearance. Sixty seven per cent (67%) of all study treated toenails evaluated in this study met the study individual toenail success criteria. The average clear nail growth was an increase in 5.18mm.* Equally important, the clinical responses observed in all four trials were achieved without a single adverse event.
DID YOU KNOW? Lunula hold many U.S. and International Patents, including US Pat 8,409,264 Fungal Infection Therapy Method with Low Level Laser
ORAL MEDICATIONS
The limitations and risks of oral anti-fungal medications have been well documented. First, treatment of the body’s most distal region – the toes – with an oral anti-fungal medication is often greeted with non-response or high rate of recurrence due to limited drug bioavailability routinely caused by insufficient blood flow. Next, the infectious agent is a eukaryote, and therefore, shares structural and biochemical similarities with our body’s eukaryotic cell. As a result, our own important biochemical pathways can be negatively affected by oral anti-fungals. Although quite rare, hepatotoxicity has been reported in patients taking oral anti-fungal medication. To mitigate the risk of liver complications, patients with specific pre-existing medical conditions cannot be prescribed oral anti-fungal medications, but for those patients who are taking anti-fungals, they must undergo routine liver function tests throughout the treatment course. Non-response, high-rate of recurrence, limited to certain patients, and serious risk of adverse events – these represent the drawback of oral anti-fungal medications.
 DID YOU KNOW? The Lunula device requires very little set-up and no operator.
DID YOU KNOW? The Lunula device requires very little set-up and no operator.
 DID YOU KNOW? Clinical Results in as little as (4) Weekly Treatments
DID YOU KNOW? Clinical Results in as little as (4) Weekly Treatments
HOW THE COMPETITION STACKS UP TO LUNULA
HISTOLOGY – LUNULA MECHANISM OF ACTION
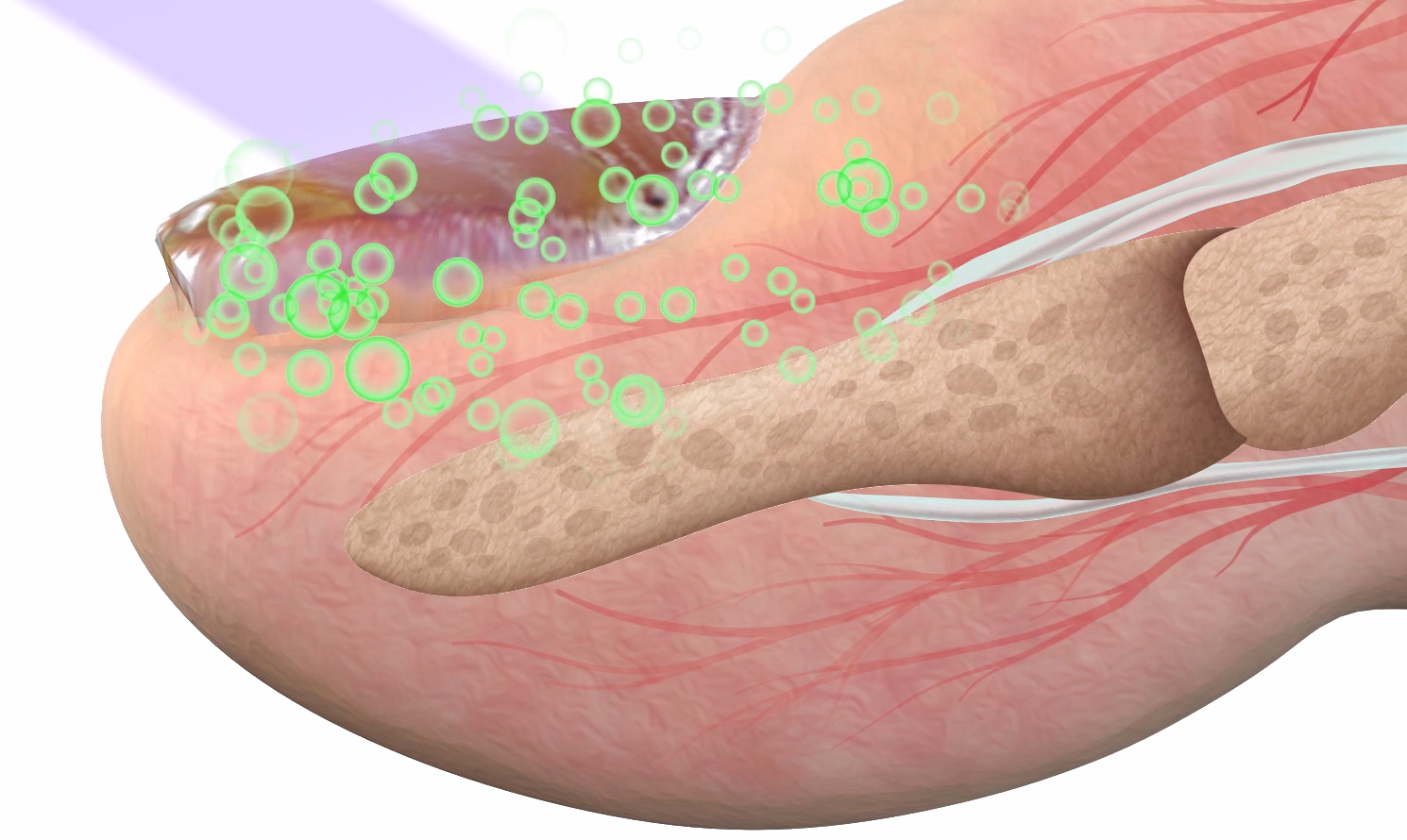
LunulaLaser is a dual-diode, low-level laser device that delivers a multifaceted treatment to target onychomycosis (OM) Lunulalaser follows the principles of photochemistry, a science that explores light’s effect cell function and behavior. The photochemical mechanism enables Lunulalaser to give a direct, non-contact treatment that produces no macroscopic sensation: no heating, tingling, burning. The mechanism of photochemistry is likened to the agonist effect of a drug, which describes the use of a certain molecule to start a secondary cascade. Laser therapy uses photonic energy to modulate secondary cellular reactions without the patient feeling the device working.
LunulaLaser impressive clinical response stems from its two therapeutic wavelengths: 405 nm (violet) and 635 nm (red). Each wavelength performs a very specific function to provide a comprehensive treatment to target OM. The manner in which the wavelengths are delivered represents an innovative and proprietary feature of LunulaLaser. Lunula administers the laser as a line-generated beam, which maximizes the treatment surface area. The fungal pathogen may not only affect multiple toes, but also may be found deep within a dystrophic nail or along the nail bed and root. The line-generated beam ensures that, regardless of where the fungal pathogen resides, an effective treatment will be administered.
405nm (VIOLET)
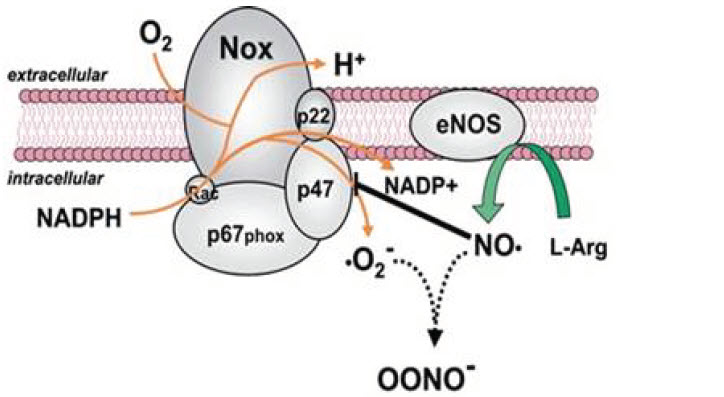
Violet has been demonstrated to have an antimicrobial effect by upregulating the production of ROS, leading to the generation of hydrogen peroxide, hypochlorous acid and droxyl radicals. When applied concurrently, the combined antimicrobial and biostimulative effects appear to provide a therapeutically beneficial combination, as demonstrated by the mean percent changes in clarity. A potential phototarget for the 405 nm wavelength is also a system responsible for catalyzing the generation of ROS, nicotinamide adenine dinucleotide phosphate oxidase (NOX). NOX transfers electrons from cytosolic NADPH to flavin adenine dinucleotide (FAD), then to extracellular molecular oxygen to generate superoxide. The third and fifth transmembrane domains of NOX bind two prosthetic heme groups that shuttle electrons from FAD to oxygen. It has been suggested that the prosthetic heme, which has been recognized as a photosensitizer, responds to the delivery of blue light. Stimulation of NOX could potentially provide two benefits: first, phagocytes are activated, and second, dermatophytes are susceptible to the toxic effects of ROS.
COMBINATION OF 635nm AND 405nm
eNOS→Nitric Oxide (NO)
Cytochrome c oxidase→Reactive Oxygen Species (ROS)